
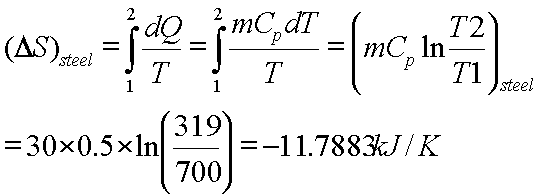
It might be thought that because the cells appear to be so much more complex than the substrate, the cells should have a lesser entropy per unit mass than the substrate. For this case, the probability of each microstate of the system is equal, so it was equivalent.
Boltzmanns paradigm was an ideal gas of N identical particles, of which Ni are in the i -th microscopic condition (range) of position and momentum. The corresponding entropy of succinic acid is 2.77 J/g deg, making it apparent that the entropy per unit mass of the cells is greater than that of the substrate. Interpreted in this way, Boltzmanns formula is the most basic formula for the thermodynamic entropy. Calculate G at 290 K for the following reaction: 2NO(g) + O 2(g) 2NO 2(g) Given.
Change in entropy formula free#
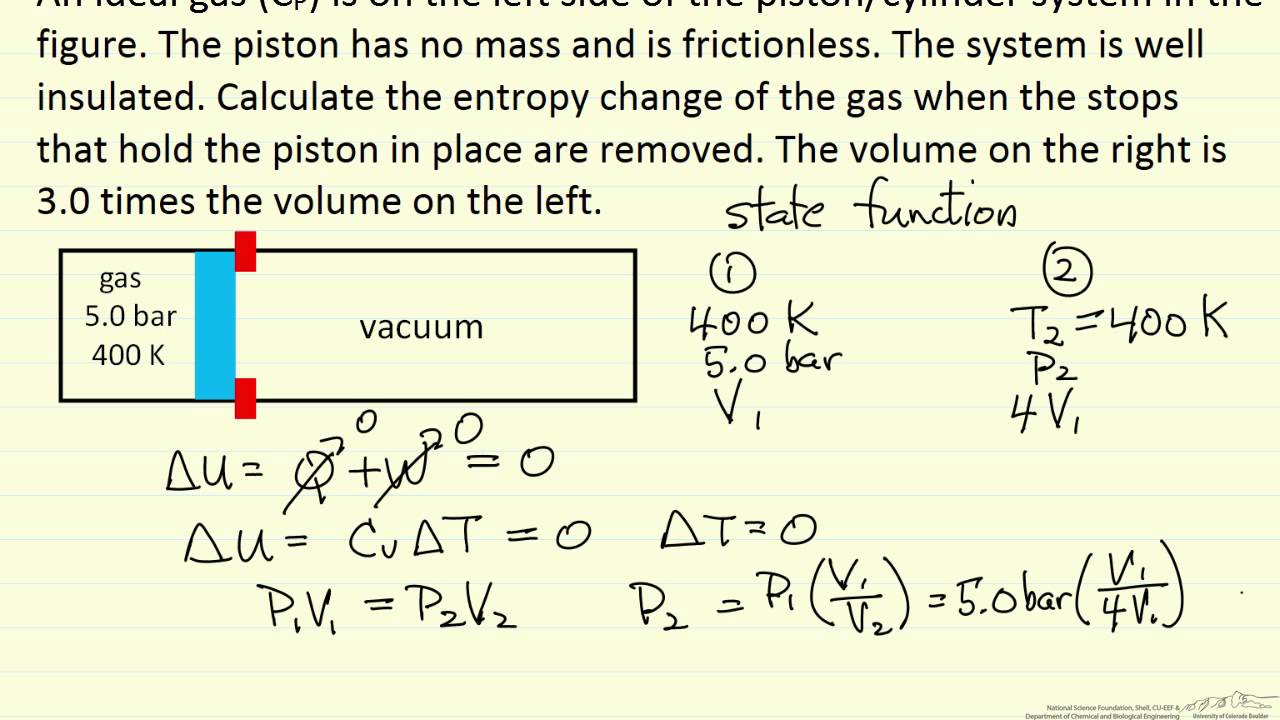
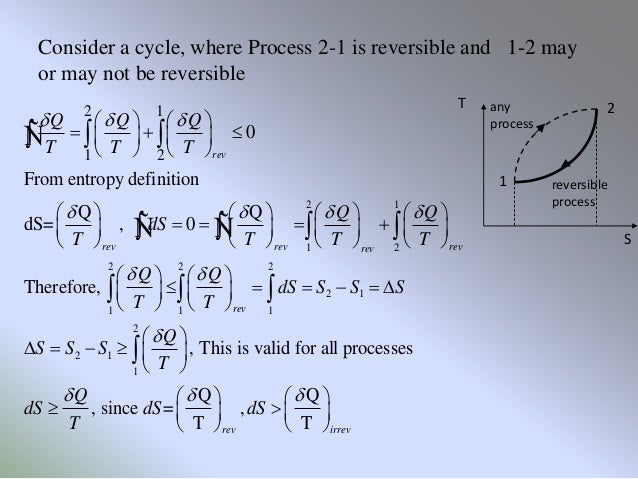
This corresponds to the statement that heat must flow from the higher. In chemical reactions involving the changes in thermodynamic quantities, a variation on this equation is often encountered: Gchange in free energy Hchange in enthalpy TS(temperature) change in entropy.
Coli K-12 cells is calculated to be 94.40 J/deg, which when divided by the mass of these cells becomes 3.90 J/g deg. The second law says that the entropy change must be equal to or greater than zero. The entropy of one unit carbon formula weight of dried E. This value could then be used to calculate the entropy change accompanying the anabolism and metabolism of succinic acid to be 30.82 J/deg and 32.40 J/mol deg, respectively. The DeltaSf of one unit carbon formula weight of Escherichia coli K-12 cells, when grown on succinic acid, was calculated to be -80.13 J/deg.


 0 kommentar(er)
0 kommentar(er)
